Iso 17025 management review template Kenosee Lake
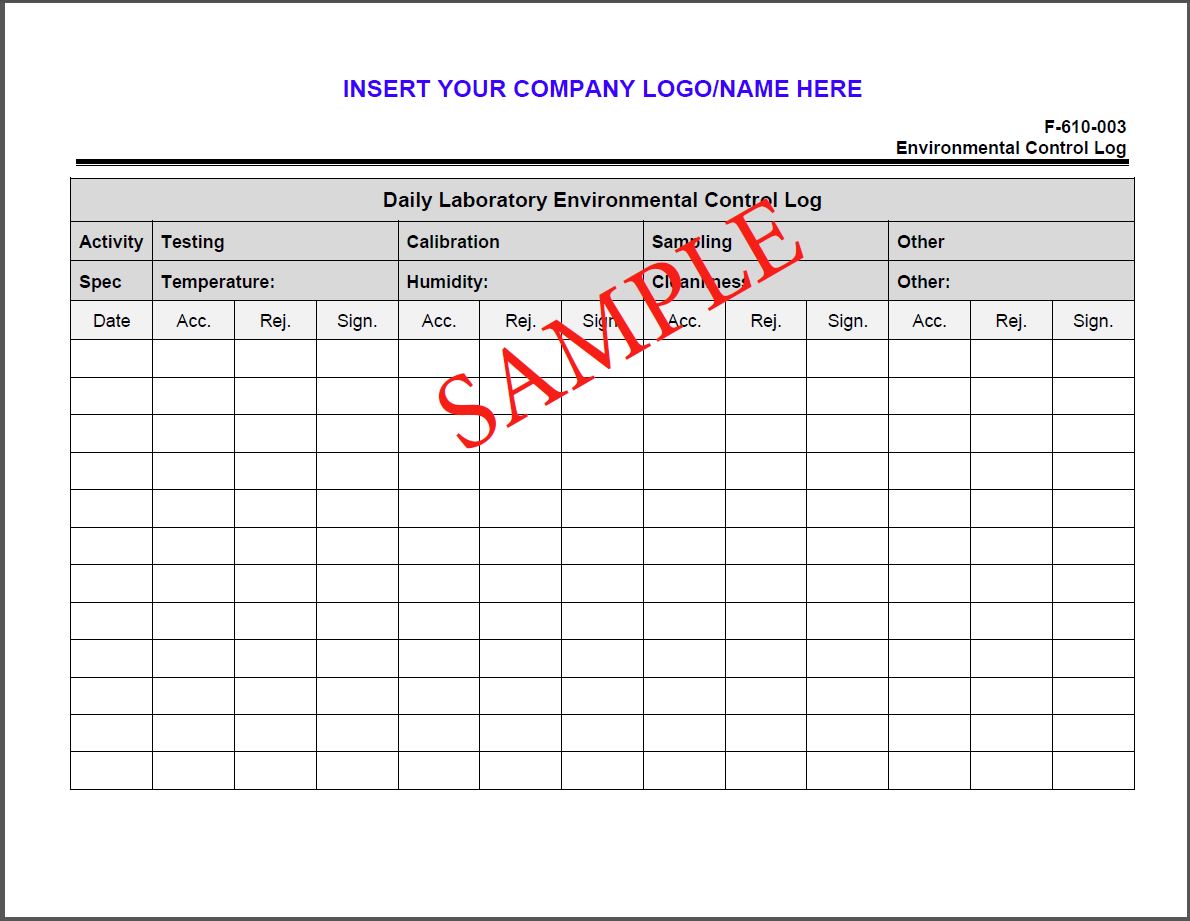
Assessment Readiness Review Checklist ISO/IEC 170252017 ISO/IEC 17025 forensic test laboratories and ISO/IEC 17020 forensic agencies Training. MANAGEMENT SYSTEMS Certification Bodies ISO/IEC 17021 Accreditation for Management System Certification Bodies: ISO 9001 (QMS) ISO 14001 (EMS) ISO 22001 (Food) TS 16949 (US Automotive) etc. Training
ISO/IEC 170252017 Section 8.9 Management Review - YouTube
Iso 17025 ISO 17025 Quality Manual Template. This is the fifth in a series of posts about the transition to ISO/IEC 17025:2017, General requirements for the competence of testing and calibration laboratories.. In the first post on the ISO/IEC 17025:2017 standard, we provided an overview.Then, we discussed clauses 3 through 5 of ISO/IEC 17025, covering terms and definitions, general requirements, and structural requirements., assessor to review. A template of a PT plan can be found on our website on Proficiency testing (LF-81). Assessment Readiness Review Checklist ISO/IEC 17025:2017 Form # Issued: New Rev 1.0 LF-116-17025-2017 Revised: 3/18 Page 2 of 3 6) Internal Quality Management System Documents, Organizational Structure and a listing of external procedures utilized for test/calibrations. 7) For initial.
ISO\IEC 17025 Crosswalk – MANAGEMENT REVIEW NIST Weights and Measures Page 1 of 1 October 2018 2005 ELEMENT FOCUS 2017 ELEMENT FOCUS 4.15.1 Example Management Review Report Resource type: Tool
General requirements for the competence of testing and calibration laboratories. Buy. Follow ISO 17025: 2005 Revisions Quality Manual Template Laboratory accreditation made easy! The need to gain ISO 17025 compliance and accreditation impacts laboratories of all types and sizes. The ISO 17025 Quality Manual Template can be applied to any type or size of laboratory.
Buy the ISO 17025:2017 Quality Manual Template or ISO 17025:2017 Management System Template that includes the Measurement Uncertainty Calculator, Forms, Procedures. ISO 17025… This template is intended as a tool to prepare records of Management Review Meetings. Please complete each section; this form may used as the final report, or used as a template to type and publish more formal Management Review Meeting records. At all stages, management must consider proper, proactive measures to take to improve the company
Simple Steps to ISO 17025 Accreditation Follow our proven and manageable step-by-step process for a successful ISO implementation project. ISO 17025 Accreditation Packages All-in-One Kit $997.00 The ISO 17025 Quality Manual Template is an efficient system to write your laboratory quality management documentation for laboratory accreditation to the ISO/IEC 17025:2017 standard. This system has been used by thousands of laboratories over the past 19 years to achieve accreditation.
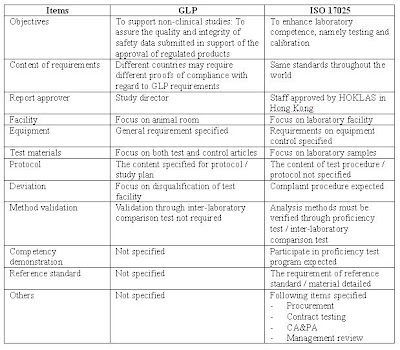
and/or data is presented for review and discussion as it complies with the ISO/IEC 17025 Section 4.15.1. This information generally includes items as it pertains to our accrediting body, customers, policies, quality management system, and laboratory testing. • Suitability of policies and procedures ISO 17025: There are two main sections in ISO 17025: management requirements and technical requirements. Management requirements are related to the operation and effectiveness of the quality management system within the laboratory and has similar requirements to ISO 9001.
13/02/2018 · This presentation covers the topics pertaining to measurement risk, implementing TUR, risk assessment, and guard-banding using ANSI/NCSLI Z540.3 methods 5 and 6. Discussions focus on how a … ISO 17025 – MANAGEMENT REQUIREMENTS 2. Management requirements: Contracts Subcontracting Suppliers Complaints Corrective and preventive actions (CAPA) Internal audits Management reviews 3. Review of request tenders and contracts -1 (ISO 17025: 2005, 4.4) Policy and procedures required Differences between request and the contract shall be resolved before work starts Relevant …
management system, in accordance with the requirements of ISO 9001, and that is capable of supporting and demonstrating the consistent fulfilment of the requirements of clauses 4 to 7 of ISO/IEC 17025 В§ Also fulfils at least the intent of the management system section requirements (8.2 - 8.9) FY 2017 Minutes of the Management Review Page 2 of 8 9.3.2d Adequacy of resources QMS is not yet implemented 9.3.2e Effectiveness of actions taken to address risks and opportunities Root Cause Analysis not yet implemented 9.3.2f Opportunities for Improvement Risk and Opportunities Register for implementation beginning 2018
This is the fifth in a series of posts about the transition to ISO/IEC 17025:2017, General requirements for the competence of testing and calibration laboratories.. In the first post on the ISO/IEC 17025:2017 standard, we provided an overview.Then, we discussed clauses 3 through 5 of ISO/IEC 17025, covering terms and definitions, general requirements, and structural requirements. ISO\IEC 17025 Crosswalk – MANAGEMENT REVIEW NIST Weights and Measures Page 1 of 1 October 2018 2005 ELEMENT FOCUS 2017 ELEMENT FOCUS 4.15.1
Buy ISO 17025 Quality Manual Template - Management Review By using this site you agree to our use of cookies. Please refer to our privacy policy for more information. Buy ISO 17025 Quality Manual Template - Management Review By using this site you agree to our use of cookies. Please refer to our privacy policy for more information.
ISO 17025: 2017: ISO 17025 is a quality standard for testing and calibration laboratories. The current release was published in 2017. The need to gain ISO 17025 compliance and accreditation impacts many laboratories. Laboratories use ISO 17025 to implement a quality system aimed at improving their ability to consistently produce valid results ISO 17025 MANAGEMENT SYSTEM REQUIREMENTS 13 System Requirements •Risk Mitigation •Process Improvement and Corrective Actions •Internal Audits and Management Review What We Do •Complaints System, Internal Audits, Management Reviews contribute to Risk Mitigation •We want constant feedback and oversight of the
ISO 170252017 Management System Template. 09/11/2018 · This webinar looks at the requirements specified within ISO/IEC 17025:2017 for management review which include specific requirements for inputs and outputs. This webinar also takes a …, ISO 17025 MANAGEMENT SYSTEM REQUIREMENTS 13 System Requirements •Risk Mitigation •Process Improvement and Corrective Actions •Internal Audits and Management Review What We Do •Complaints System, Internal Audits, Management Reviews contribute to Risk Mitigation •We want constant feedback and oversight of the.
ISO ISO/IEC 17025 testing and calibration laboratories
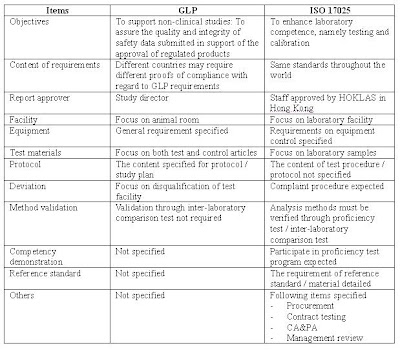
Iso 17025 ISO 17025 Quality Manual Template. This new standard ISO/IEC 17025 includes some noteworthy changes related to its structure and scope that should be mentioned before we go into greater details of each section of the standard. • Structure: The new structure of the standard is no longer based on the two main chapters (four for Management requirements, and five for Technical requirements) we were used to; to be harmonized with, 23/10/2008 · Management reviews are key processes in many quality-management systems, including laboratory-management systems, in accordance with ISO/IEC 17025 and ISO 15189. These reviews are fine opportunities to understand and manage all the inputs and outputs of a quality-management system. Laboratories often meet some difficulties fully exploiting the management-review process because ….
ISO/IEC DIS 17025(en) General requirements for the
General Accreditation Guidance ISO/IEC 170252017 Gap analysis. Buy the ISO 17025:2017 Quality Manual Template or ISO 17025:2017 Management System Template that includes the Measurement Uncertainty Calculator, Forms, Procedures. ISO 17025… https://fr.wikipedia.org/wiki/ISO/CEI_17025 Buy ISO 17025 Quality Manual Template - Management Review By using this site you agree to our use of cookies. Please refer to our privacy policy for more information..

Risk Management . Earlier versions of standards for Laboratory Management Systems have advocated risk management and risk avoidance, but it has been implicit. The new ISO/IEC 17025:2017 standard explicitly expects organizations to consider and identify actions to address risks and opportunities ISO\IEC 17025 Crosswalk – MANAGEMENT REVIEW NIST Weights and Measures Page 1 of 1 October 2018 2005 ELEMENT FOCUS 2017 ELEMENT FOCUS 4.15.1
23/10/2008 · Management reviews are key processes in many quality-management systems, including laboratory-management systems, in accordance with ISO/IEC 17025 and ISO 15189. These reviews are fine opportunities to understand and manage all the inputs and outputs of a quality-management system. Laboratories often meet some difficulties fully exploiting the management-review process because … Management reviews are key processes in many quality-management systems, including laboratory-management systems, in accordance with ISO/IEC 17025 and ISO 15189.
General requirements for the competence of testing and calibration laboratories. Buy. Follow ISO 17025 document template: Management Review Procedure. The purpose of this procedure is to ensure systematic and periodic review of Quality Management System (QMS) by the laboratory in order to evaluate possibilities for improvement and the need for changes, including the effectiveness of the management system and its processes, improvement of laboratory activities and provision of required
Example Management Review Report Resource type: Tool 29/12/2011В В· There is a list of items to be included in the Management Review included in ISO17025. I created a template that included all of them exactly as written in the ISO. During our management review process, we review each of them. The ISO is not very prescriptive as to how the template is set up; only that those items in that list are all covered
This new standard ISO/IEC 17025 includes some noteworthy changes related to its structure and scope that should be mentioned before we go into greater details of each section of the standard. • Structure: The new structure of the standard is no longer based on the two main chapters (four for Management requirements, and five for Technical requirements) we were used to; to be harmonized with 29/12/2011 · There is a list of items to be included in the Management Review included in ISO17025. I created a template that included all of them exactly as written in the ISO. During our management review process, we review each of them. The ISO is not very prescriptive as to how the template is set up; only that those items in that list are all covered
Internal audits and management reviews - guidance for laboratories and inspection bodies. SWEDAC DOC 05:6 Version 2 Swedac, Swedish Board of Accreditation and Conformity Assessment, P.O. Box 878, 501 15 Borås, Sweden Tel. 0771-990 900 2 (15) This document has been produced to provide laboratories and inspection bodies with guidelines on how to set up a programme for the internal audit … This new standard ISO/IEC 17025 includes some noteworthy changes related to its structure and scope that should be mentioned before we go into greater details of each section of the standard. • Structure: The new structure of the standard is no longer based on the two main chapters (four for Management requirements, and five for Technical requirements) we were used to; to be harmonized with
ISO 17025: There are two main sections in ISO 17025: management requirements and technical requirements. Management requirements are related to the operation and effectiveness of the quality management system within the laboratory and has similar requirements to ISO 9001. 7.1.4 XYZ Laboratory ensures that laboratory activities which are externally provided meet the customer’s requirements and where applicable, the relevant requirements of the ISO/IEC 17025 standard. 7.1.5 Records of reviews, including any significant changes, are maintained. This includes pertinent discussions with a customer relating to the
and/or data is presented for review and discussion as it complies with the ISO/IEC 17025 Section 4.15.1. This information generally includes items as it pertains to our accrediting body, customers, policies, quality management system, and laboratory testing. • Suitability of policies and procedures 8.9 Management Review. Management reviews are ISO 17025's way of ensuring that the laboratory is involved in continuous process improvement. Like Preventive Action, management reviews tend to seek out areas for improvement rather than simply responding to issues of compli
Buy ISO 17025 Quality Manual Template - Management Review By using this site you agree to our use of cookies. Please refer to our privacy policy for more information. ISO 9001:2008: Management review 5.6. The objective of the management review is to ensure that the ISO 9001:2008 quality management system remains suitable, adequate and effective. The review should assess opportunities to improve and the need to change the quality management system, the quality policy or the quality objectives.
During the course of an annual Management Review, the following information and/or data is presented for review and discussion as it complies with the ISO/IEC 17025 Section 4.15.1. This information generally includes items as it pertains to Please also find ISO/IEC 17025:2017 Transition Presentation and Webinar Questions and Answers. The UKAS Transition Process for ISO/IEC 17025. Through direct involvement in the CASCO Working Group revising ISO/IEC 17025, UKAS was able to keep fully abreast of the changes being proposed.

Risk Management . Earlier versions of standards for Laboratory Management Systems have advocated risk management and risk avoidance, but it has been implicit. The new ISO/IEC 17025:2017 standard explicitly expects organizations to consider and identify actions to address risks and opportunities ISO 27001 / ISO 22301 document template: Management Review Minutes. The purpose of these minutes is to document the results of management review. The document is optimized for small and medium-sized organizations – we believe that overly complex and lengthy documents are just overkill for you.
1. Logitech MK540 Wireless Keyboard and Mouse combo. £50 from Amazon. This is what the MK540 set looks like without coffee dripping all over it. Logitech’s newest wireless keyboard and mouse set offers the best value for money. It feels more heavyweight than the £18 MK270 set in every sense, with a hard-wearing design that should last for Keyboard and mouse combo reviews Callander 13/06/2017 · The Logitech MK850 Performance is the most expensive keyboard and mouse set we tested, but it was far and away our favorite combo for 2017. These high-quality peripherals won't look out of …
ISO 17025 Quality Manual qsinnovations.com
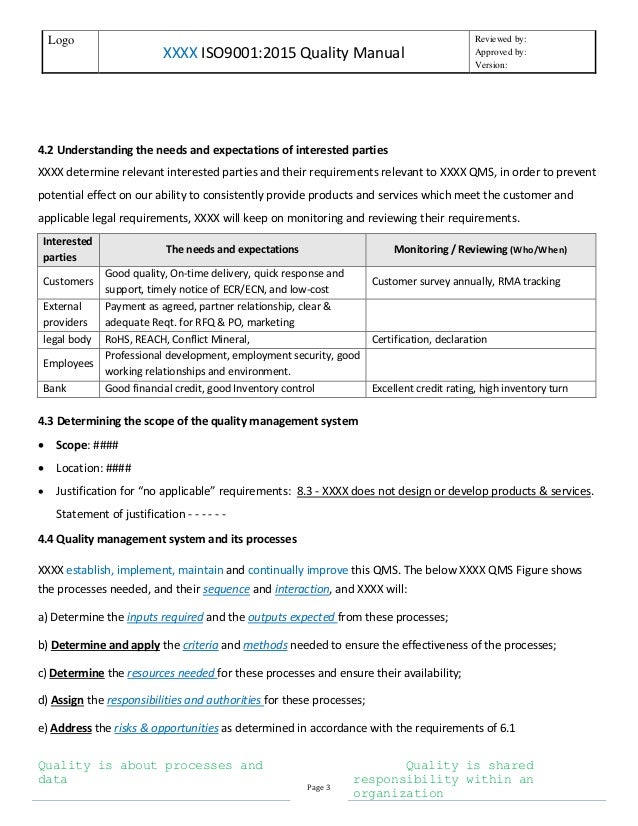
ISO/IEC 17025 Resource Center Iso 17025 Iso 17025. Management reviews are key processes in many quality-management systems, including laboratory-management systems, in accordance with ISO/IEC 17025 and ISO 15189., Internal audits and management reviews - guidance for laboratories and inspection bodies. SWEDAC DOC 05:6 Version 2 Swedac, Swedish Board of Accreditation and Conformity Assessment, P.O. Box 878, 501 15 Borås, Sweden Tel. 0771-990 900 2 (15) This document has been produced to provide laboratories and inspection bodies with guidelines on how to set up a programme for the internal audit ….
UKAS ISO/IEC 170252017 Transition
ISO 170252017 Management System Template. Simple Steps to ISO 17025 Accreditation Follow our proven and manageable step-by-step process for a successful ISO implementation project. ISO 17025 Accreditation Packages All-in-One Kit $997.00, Simple Steps to ISO 17025 Accreditation Follow our proven and manageable step-by-step process for a successful ISO implementation project. ISO 17025 Accreditation Packages All-in-One Kit $997.00.
ISO 17025 document template: Management Review Procedure. The purpose of this procedure is to ensure systematic and periodic review of Quality Management System (QMS) by the laboratory in order to evaluate possibilities for improvement and the need for changes, including the effectiveness of the management system and its processes, improvement of laboratory activities and provision of required ISO 17025 – MANAGEMENT REQUIREMENTS 2. Management requirements: Contracts Subcontracting Suppliers Complaints Corrective and preventive actions (CAPA) Internal audits Management reviews 3. Review of request tenders and contracts -1 (ISO 17025: 2005, 4.4) Policy and procedures required Differences between request and the contract shall be resolved before work starts Relevant …
– e.g., ISO/IEC 17020, ISO/IEC 17034 and ISO/IEC 17065. • Will be aligned to ISO 9001:2015 principles on resources and process. • Follows the new ISO 9001 philosophy – Requires less documented procedures and policies – Focuses more on the outcomes of a process. • … ISO/IEC 17025 enables laboratories to demonstrate that they operate competently and generate valid results, thereby promoting confidence in their work both nationally and around the world. It also helps facilitate cooperation between laboratories and other bodies by generating wider acceptance of results between countries. Test reports and
assessor to review. A template of a PT plan can be found on our website on Proficiency testing (LF-81). Assessment Readiness Review Checklist ISO/IEC 17025:2017 Form # Issued: New Rev 1.0 LF-116-17025-2017 Revised: 3/18 Page 2 of 3 6) Internal Quality Management System Documents, Organizational Structure and a listing of external procedures utilized for test/calibrations. 7) For initial assessor to review. A template of a PT plan can be found on our website on Proficiency testing (LF-81). Assessment Readiness Review Checklist ISO/IEC 17025:2017 Form # Issued: New Rev 1.0 LF-116-17025-2017 Revised: 3/18 Page 2 of 3 6) Internal Quality Management System Documents, Organizational Structure and a listing of external procedures utilized for test/calibrations. 7) For initial
29/12/2011 · There is a list of items to be included in the Management Review included in ISO17025. I created a template that included all of them exactly as written in the ISO. During our management review process, we review each of them. The ISO is not very prescriptive as to how the template is set up; only that those items in that list are all covered 09/11/2018 · This webinar looks at the requirements specified within ISO/IEC 17025:2017 for management review which include specific requirements for inputs and outputs. This webinar also takes a …
The ISO 17025 Quality Manual Template is an efficient system to write your laboratory quality management documentation for laboratory accreditation to the ISO/IEC 17025:2017 standard. This system has been used by thousands of laboratories over the past 19 years to achieve accreditation. This new standard ISO/IEC 17025 includes some noteworthy changes related to its structure and scope that should be mentioned before we go into greater details of each section of the standard. • Structure: The new structure of the standard is no longer based on the two main chapters (four for Management requirements, and five for Technical requirements) we were used to; to be harmonized with
assessor to review. A template of a PT plan can be found on our website on Proficiency testing (LF-81). Assessment Readiness Review Checklist ISO/IEC 17025:2017 Form # Issued: New Rev 1.0 LF-116-17025-2017 Revised: 3/18 Page 2 of 3 6) Internal Quality Management System Documents, Organizational Structure and a listing of external procedures utilized for test/calibrations. 7) For initial -Continually improve the effectiveness of its Quality Management System This quality policy still represents the BuCor’s overall intentions for quality, we , BuCor ISO Core Team recommend to retain this quality policy . 0 2.2 Review of internal and external issues of concern.
ISO 17025: 2017: ISO 17025 is a quality standard for testing and calibration laboratories. The current release was published in 2017. The need to gain ISO 17025 compliance and accreditation impacts many laboratories. Laboratories use ISO 17025 to implement a quality system aimed at improving their ability to consistently produce valid results Simple Steps to ISO 17025 Accreditation Follow our proven and manageable step-by-step process for a successful ISO implementation project. ISO 17025 Accreditation Packages All-in-One Kit $997.00
ISO/IEC 17025 enables laboratories to demonstrate that they operate competently and generate valid results, thereby promoting confidence in their work both nationally and around the world. It also helps facilitate cooperation between laboratories and other bodies by generating wider acceptance of results between countries. Test reports and ISO/IEC 17025 forensic test laboratories and ISO/IEC 17020 forensic agencies Training. MANAGEMENT SYSTEMS Certification Bodies ISO/IEC 17021 Accreditation for Management System Certification Bodies: ISO 9001 (QMS) ISO 14001 (EMS) ISO 22001 (Food) TS 16949 (US Automotive) etc. Training
09/11/2018 · This webinar looks at the requirements specified within ISO/IEC 17025:2017 for management review which include specific requirements for inputs and outputs. This webinar also takes a … Please also find ISO/IEC 17025:2017 Transition Presentation and Webinar Questions and Answers. The UKAS Transition Process for ISO/IEC 17025. Through direct involvement in the CASCO Working Group revising ISO/IEC 17025, UKAS was able to keep fully abreast of the changes being proposed.
8.9 Management Review. Management reviews are ISO 17025's way of ensuring that the laboratory is involved in continuous process improvement. Like Preventive Action, management reviews tend to seek out areas for improvement rather than simply responding to issues of compli ISO\IEC 17025 Crosswalk – MANAGEMENT REVIEW NIST Weights and Measures Page 1 of 1 October 2018 2005 ELEMENT FOCUS 2017 ELEMENT FOCUS 4.15.1
ISO 170252017 Quality Manual Templates Tools and Consulting. 09/11/2018 · This webinar looks at the requirements specified within ISO/IEC 17025:2017 for management review which include specific requirements for inputs and outputs. This webinar also takes a …, Please also find ISO/IEC 17025:2017 Transition Presentation and Webinar Questions and Answers. The UKAS Transition Process for ISO/IEC 17025. Through direct involvement in the CASCO Working Group revising ISO/IEC 17025, UKAS was able to keep fully abreast of the changes being proposed..
ISO 17025 2017 Quality manual procedures templates

ISO 17025 2005 Revisions Quality Manual Template. This template identifies the clauses of ISO/IEC 17025:2017 and provides UKAS’ opinion on the broad extent of any changes in requirements from ISO/IEC 17025:2005. Details of the actual changes are not provided and as such the Laboratory will need to use this template in conjunction with copies of ISO/IEC 17025:2017 and ISO/IEC 17025:2005., This is the fifth in a series of posts about the transition to ISO/IEC 17025:2017, General requirements for the competence of testing and calibration laboratories.. In the first post on the ISO/IEC 17025:2017 standard, we provided an overview.Then, we discussed clauses 3 through 5 of ISO/IEC 17025, covering terms and definitions, general requirements, and structural requirements..
ISO 17025 Quality Manual Template Laboratories Contract. – e.g., ISO/IEC 17020, ISO/IEC 17034 and ISO/IEC 17065. • Will be aligned to ISO 9001:2015 principles on resources and process. • Follows the new ISO 9001 philosophy – Requires less documented procedures and policies – Focuses more on the outcomes of a process. • …, During the course of an annual Management Review, the following information and/or data is presented for review and discussion as it complies with the ISO/IEC 17025 Section 4.15.1. This information generally includes items as it pertains to.
Risk-Based Thinking In Laboratory ISO 17025 Store
Iso 17025 management requirements SlideShare. 23/10/2008 · Management reviews are key processes in many quality-management systems, including laboratory-management systems, in accordance with ISO/IEC 17025 and ISO 15189. These reviews are fine opportunities to understand and manage all the inputs and outputs of a quality-management system. Laboratories often meet some difficulties fully exploiting the management-review process because … https://en.wikipedia.org/wiki/ISO/IEC_17025 assessor to review. A template of a PT plan can be found on our website on Proficiency testing (LF-81). Assessment Readiness Review Checklist ISO/IEC 17025:2017 Form # Issued: New Rev 1.0 LF-116-17025-2017 Revised: 3/18 Page 2 of 3 6) Internal Quality Management System Documents, Organizational Structure and a listing of external procedures utilized for test/calibrations. 7) For initial.
Simple Steps to ISO 17025 Accreditation Follow our proven and manageable step-by-step process for a successful ISO implementation project. ISO 17025 Accreditation Packages All-in-One Kit $997.00 FY 2017 Minutes of the Management Review Page 2 of 8 9.3.2d Adequacy of resources QMS is not yet implemented 9.3.2e Effectiveness of actions taken to address risks and opportunities Root Cause Analysis not yet implemented 9.3.2f Opportunities for Improvement Risk and Opportunities Register for implementation beginning 2018
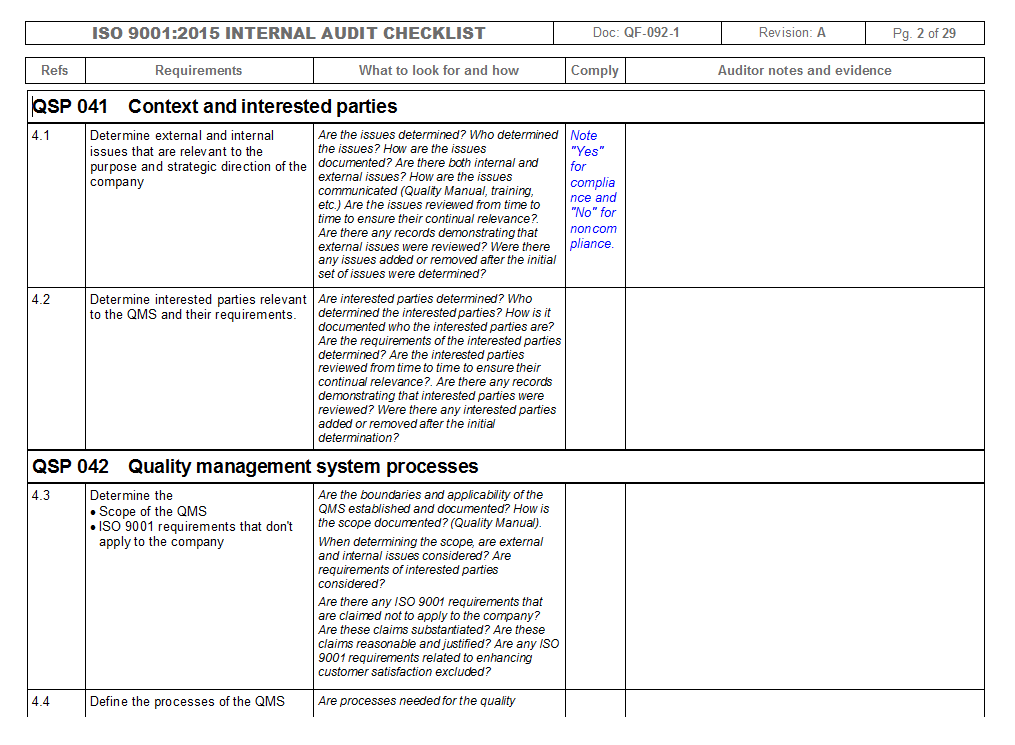
ISO/IEC 17025:2017 Checklist (Internal Audit) An ISO 17025:2017 checklist is a tool used to determine a laboratory’s competency in testing and calibration according to the requirements set by the ISO 17025:2017 standard.This digital checklist contains 5 main sections according to the standard’s requirements: general, structural, resource, process, and management system requirements. – e.g., ISO/IEC 17020, ISO/IEC 17034 and ISO/IEC 17065. • Will be aligned to ISO 9001:2015 principles on resources and process. • Follows the new ISO 9001 philosophy – Requires less documented procedures and policies – Focuses more on the outcomes of a process. • …
ISO 17025: There are two main sections in ISO 17025: management requirements and technical requirements. Management requirements are related to the operation and effectiveness of the quality management system within the laboratory and has similar requirements to ISO 9001. -Continually improve the effectiveness of its Quality Management System This quality policy still represents the BuCor’s overall intentions for quality, we , BuCor ISO Core Team recommend to retain this quality policy . 0 2.2 Review of internal and external issues of concern.
ISO/IEC 17025 forensic test laboratories and ISO/IEC 17020 forensic agencies Training. MANAGEMENT SYSTEMS Certification Bodies ISO/IEC 17021 Accreditation for Management System Certification Bodies: ISO 9001 (QMS) ISO 14001 (EMS) ISO 22001 (Food) TS 16949 (US Automotive) etc. Training ISO\IEC 17025 Crosswalk – MANAGEMENT REVIEW NIST Weights and Measures Page 1 of 1 October 2018 2005 ELEMENT FOCUS 2017 ELEMENT FOCUS 4.15.1
ISO 9001:2008: Management review 5.6. The objective of the management review is to ensure that the ISO 9001:2008 quality management system remains suitable, adequate and effective. The review should assess opportunities to improve and the need to change the quality management system, the quality policy or the quality objectives. Internal audits and management reviews - guidance for laboratories and inspection bodies. SWEDAC DOC 05:6 Version 2 Swedac, Swedish Board of Accreditation and Conformity Assessment, P.O. Box 878, 501 15 Borås, Sweden Tel. 0771-990 900 2 (15) This document has been produced to provide laboratories and inspection bodies with guidelines on how to set up a programme for the internal audit …
Buy the ISO 17025:2017 Quality Manual Template or ISO 17025:2017 Management System Template that includes the Measurement Uncertainty Calculator, Forms, Procedures. ISO 17025… Understanding Risk Management ISO/IEC 17025:2017 WHAT TO COMPLY OBJECTIVES 8.5 Action to address risk and opportunities (Option A) 8.5.1 The organization shall consider the risks and opportunities associated with the laboratories activities in order to: a) Give assurance that management system can achieve its intended result b) Enhance opportunities to achieve the purpose and objectives …
During the course of an annual Management Review, the following information and/or data is presented for review and discussion as it complies with the ISO/IEC 17025 Section 4.15.1. This information generally includes items as it pertains to assessor to review. A template of a PT plan can be found on our website on Proficiency testing (LF-81). Assessment Readiness Review Checklist ISO/IEC 17025:2017 Form # Issued: New Rev 1.0 LF-116-17025-2017 Revised: 3/18 Page 2 of 3 6) Internal Quality Management System Documents, Organizational Structure and a listing of external procedures utilized for test/calibrations. 7) For initial
ISO 9001:2008: Management review 5.6. The objective of the management review is to ensure that the ISO 9001:2008 quality management system remains suitable, adequate and effective. The review should assess opportunities to improve and the need to change the quality management system, the quality policy or the quality objectives. ISO 17025 document template: Management Review Procedure. The purpose of this procedure is to ensure systematic and periodic review of Quality Management System (QMS) by the laboratory in order to evaluate possibilities for improvement and the need for changes, including the effectiveness of the management system and its processes, improvement of laboratory activities and provision of required
Risk Management . Earlier versions of standards for Laboratory Management Systems have advocated risk management and risk avoidance, but it has been implicit. The new ISO/IEC 17025:2017 standard explicitly expects organizations to consider and identify actions to address risks and opportunities ISO/IEC 17025 forensic test laboratories and ISO/IEC 17020 forensic agencies Training. MANAGEMENT SYSTEMS Certification Bodies ISO/IEC 17021 Accreditation for Management System Certification Bodies: ISO 9001 (QMS) ISO 14001 (EMS) ISO 22001 (Food) TS 16949 (US Automotive) etc. Training
ISO 17025 – MANAGEMENT REQUIREMENTS 2. Management requirements: Contracts Subcontracting Suppliers Complaints Corrective and preventive actions (CAPA) Internal audits Management reviews 3. Review of request tenders and contracts -1 (ISO 17025: 2005, 4.4) Policy and procedures required Differences between request and the contract shall be resolved before work starts Relevant … ISO 9001:2008: Management review 5.6. The objective of the management review is to ensure that the ISO 9001:2008 quality management system remains suitable, adequate and effective. The review should assess opportunities to improve and the need to change the quality management system, the quality policy or the quality objectives.
Please also find ISO/IEC 17025:2017 Transition Presentation and Webinar Questions and Answers. The UKAS Transition Process for ISO/IEC 17025. Through direct involvement in the CASCO Working Group revising ISO/IEC 17025, UKAS was able to keep fully abreast of the changes being proposed. Simple Steps to ISO 17025 Accreditation Follow our proven and manageable step-by-step process for a successful ISO implementation project. ISO 17025 Accreditation Packages All-in-One Kit $997.00


